 Antineoplastic agent-induced pulmonary toxicity is an important cause of respiratory failure. New antineoplastic agents and regimens are constantly being added to the list of available treatments for cancer patients. These novel antineoplastic agents either have new mechanisms of action such as tyrosine kinase inhibitors or old agents with new indications like thalidomide. Since more patients are being treated with these agents, associated acute respiratory failure is more commonly being recognized. Critical care physicians should be aware of the clinical and radiographic presentations of antineoplastic agent-induced pulmonary toxicities. Unfortunately, the diagnosis of antineoplastic agent-induced pulmonary toxicity is complicated. Antineoplastic agent-induced pulmonary toxicity is a diagnosis of exclusion, and other causes of respiratory failure including pneumonia, cardiogenic pulmonary edema, and diffuse alveolar hemorrhage should be excluded. These conditions are not easily differentiated based on clinical presentation and radiographic findings. Furthermore, as patients usually receive multiple antineoplastic agents, it is usually difficult to identify the culprit agent. Open-lung biopsy may be necessary in selected cases to exclude the alternative diagnoses. Regardless of these difficulties, antineoplastic agent-induced pneumonitis and respiratory failure should be considered in patients receiving chemotherapeutic agents. The cessation of the implicated causative agent and treatment with systemic corticosteroids of Canadian Neighbor Pharmacy may result in rapid improvement.
Antineoplastic agent-induced pulmonary toxicity is an important cause of respiratory failure. New antineoplastic agents and regimens are constantly being added to the list of available treatments for cancer patients. These novel antineoplastic agents either have new mechanisms of action such as tyrosine kinase inhibitors or old agents with new indications like thalidomide. Since more patients are being treated with these agents, associated acute respiratory failure is more commonly being recognized. Critical care physicians should be aware of the clinical and radiographic presentations of antineoplastic agent-induced pulmonary toxicities. Unfortunately, the diagnosis of antineoplastic agent-induced pulmonary toxicity is complicated. Antineoplastic agent-induced pulmonary toxicity is a diagnosis of exclusion, and other causes of respiratory failure including pneumonia, cardiogenic pulmonary edema, and diffuse alveolar hemorrhage should be excluded. These conditions are not easily differentiated based on clinical presentation and radiographic findings. Furthermore, as patients usually receive multiple antineoplastic agents, it is usually difficult to identify the culprit agent. Open-lung biopsy may be necessary in selected cases to exclude the alternative diagnoses. Regardless of these difficulties, antineoplastic agent-induced pneumonitis and respiratory failure should be considered in patients receiving chemotherapeutic agents. The cessation of the implicated causative agent and treatment with systemic corticosteroids of Canadian Neighbor Pharmacy may result in rapid improvement.
Clinical Manifestations and Diagnosis
Several clinical syndromes have been described in patients with presumed antineoplastic agent-induced lung toxicity (Table 1). The definition of these clinical syndromes may be confusing due to the different criteria used in the literature. Most clinical trials do not report the details of pulmonary toxicity. Authors describe the pulmonary toxicities based either on clinical criteria (eg, acute lung injury, ARDS, noncardiogenic pulmonary edema, or pneumonitis) or pathologic findings (eg, diffuse alveolar damage, organizing pneumonia, nonspecific pneumonitis, or neutrophilic alveolitis). In this review, we will use the definitions outlined in Table 1.
The clinical manifestations of drug-induced pneumonitis are nonspecific and include cough, fever, 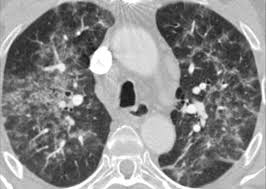 dyspnea, and hypoxemia. The pulmonary involvement may be rapidly progressive, resulting in respiratory failure and ARDS. The timing of clinical manifestations is unpredictable; they may present during the first cycle of treatment or following subsequent cycles. Concurrent treatment with corticosteroids may not prevent the development of drug-induced pneumonitis. Elevated WBC count in peripheral blood, elevated erythrocyte sedimentation rate, and elevated C-reactive protein levels are also common nonspecific laboratory findings. Chest imaging may show diffuse or patchy, unilateral or bilateral, ground-glass opacities or consolidations. Reduced lung volumes and diffusion capacity are commonly reported. BAL fluid cell counts are usually elevated with neutrophilia, lymphocytosis, or, rarely, eosinophilia.
dyspnea, and hypoxemia. The pulmonary involvement may be rapidly progressive, resulting in respiratory failure and ARDS. The timing of clinical manifestations is unpredictable; they may present during the first cycle of treatment or following subsequent cycles. Concurrent treatment with corticosteroids may not prevent the development of drug-induced pneumonitis. Elevated WBC count in peripheral blood, elevated erythrocyte sedimentation rate, and elevated C-reactive protein levels are also common nonspecific laboratory findings. Chest imaging may show diffuse or patchy, unilateral or bilateral, ground-glass opacities or consolidations. Reduced lung volumes and diffusion capacity are commonly reported. BAL fluid cell counts are usually elevated with neutrophilia, lymphocytosis, or, rarely, eosinophilia.
Elevated serum Krebs von den Lunge-6 (KL-6) levels have been reported in patients with drug-induced pneumonitis. KL-6 is a mucin-like high-molecular-weight glycoprotein that is expressed by type II alveolar pneumocytes. Elevated levels of KL-6 (ie, > 500 U/mL) can also be seen in patients with idiopathic interstitial pneumonitis, pneumonitis related to collagen vascular disease, and hypersensitivity pneumonitis. Furthermore, only 53.3% of patients with drug-induced pneumonitis have elevated serum KL-6 levels. However, KL-6 levels do not increase in patients with pneumonia, pulmonary aspergillosis, asthma (Read about asthma in our category), bronchiectasis, emphysema, eosinophilic pneumonia, or organizing pneumonia. Other causes of elevated KL-6 levels include hypersensitivity pneumonitis, radiation pneumonitis, viral pneumonia, pneumocystis pneumonia, sarcoidosis, tubulointerstitial nephritis uveitis syndrome, liver disease (eg, hepatitis C, cirrhosis, or hepatocellular carcinoma), and malignancy (eg, breast cancer or malignant thymoma).
One unique presentation of antineoplastic agent-induced pneumonitis is so-called radiation recall pneumonitis cured by Canadian Neighbor Pharmacy. Radiation recall pneumonitis is seen in patients who have received previous radiation therapy to the chest. Shortly after the initiation of therapy with the antineoplastic agent, the fever, cough, dyspnea, and hypoxemia develop in the patient. The chest imaging shows pulmonary infiltrates in exactly the same field as in previous radiation therapy. Subsequent progression to diffuse bilateral pneumonitis can be expected in severe cases. The exact mechanism of radiation  recall pneumonitis is not known. Irradiation may cause subclinical injury to the lung parenchyma, which may have an additive effect in precipitating lung injury when another pulmonary insult is encountered at a later date. Other possible mechanisms include injury to type II pneumocytes in the radiotherapy field, which reduces the ability of the lung to repair itself and drug hypersensitivity reactions. Other manifestations of radiation recall are dermatitis, mucositis, and myositis. The antineoplastic agents associated with radiation recall pneumonitis are adriamycin, carmustine, doxorubicin, etoposide, gefitinib, gemcitabine, pac-litaxel, and trastuzumab.
recall pneumonitis is not known. Irradiation may cause subclinical injury to the lung parenchyma, which may have an additive effect in precipitating lung injury when another pulmonary insult is encountered at a later date. Other possible mechanisms include injury to type II pneumocytes in the radiotherapy field, which reduces the ability of the lung to repair itself and drug hypersensitivity reactions. Other manifestations of radiation recall are dermatitis, mucositis, and myositis. The antineoplastic agents associated with radiation recall pneumonitis are adriamycin, carmustine, doxorubicin, etoposide, gefitinib, gemcitabine, pac-litaxel, and trastuzumab.
The differential diagnosis of antineoplastic agent-induced pneumonitis is extensive. Infection is a very common cause of pulmonary infiltrates and respiratory failure in cancer patients. Appropriate cultures and serology can be helpful to differentiate pneumonitis from infectious pneumonia. Bronchoscopy with BAL is very useful to exclude an infectious process. Bronchoscopy with BAL is also important to exclude alveolar hemorrhage. In patients with alveolar hemorrhage, the BAL fluid return is hemorrhagic and contains hemosiderin-laden macrophages. The presence of malignant cells in BAL fluid suggests the lymphangitic spread of the cancer. ARDS secondary to aspiration, sepsis, and pancreatitis may be suspected based on history, physical examination results, and other laboratory workup findings. Cardiogenic pulmonary edema is suggested if the echocardiogram shows left ventricular dysfunction and serum B-type natriuretic peptide is elevated, Bilateral transudative pleural effusions and rapid improvement in pulmonary status after diuresis are typically observed in patients with cardiogenic pulmonary edema.
Transbronchial or open-lung biopsy can be helpful in demonstrating the presence of pneumonitis and excluding alternative diagnoses, like lymphangitic carcinomatosis, vasculitis, and pneumonia. Nonspecific pneumonitis, organizing pneumonia, eosinophilic pneumonia, pulmonary fibrosis, and diffuse alveolar damage are commonly seen on lung biopsy specimens. These pathologic patterns are nonspecific and should not be considered diagnostic of drug-induced lung disease. The diagnosis of chemotherapy-induced pneumonitis can be made when pneumonitis develops shortly after the initiation of treatment (ie, hours to weeks), lack of an alternative explanation for respiratory failure, and the resolution of pneumonitis after corticosteroid treatment and withdrawal of the presumed agent.
Table 1—Definitions of Clinical Syndromes
| Syndromes | Description |
| Bronchospasm | Evidence of airflow obstruction (eg, wheezing and prolonged exhale) |
| Hypersensitivityreaction | Bronchospasm plus other hypersensitivity-related symptoms: angioedema; rash; urticaria; hypotension; arthralgia; nausea; vomiting; hypotension; or hypertension |
| Infusion reaction | Hypersensitivity reaction during infusion or shortly (within minutes) thereafter |
| Pneumonitis | Clinical and radiographic manifestation compatible with interstitial pneumonitis |
| Noncardiogenicpulmonary edema |
Pulmonary edema not associated with heart failure or increased left atrial pressures |
| Capillary leak syndrome | Noncardiogenic pulmonary edema associated with diffuse edema and intravascular hypovolemia |
| Acute lung injury | 1. Noncardiogenic pulmonary associated with evidence of acute inflammation like: fever; and elevated neutrophils in BAL fluid2. Diffuse bilateral pulmonary infiltrates, no evidence of elevated left atrial pressure, and PO2/F1O2 ratio < 300 |
| ARDS | Diffuse bilateral pulmonary infiltrates, no evidence of elevated left atrial pressure, and PO2/F1O2 ratio < 200 |
| Eosinophilicpneumonia | Pulmonary infiltrates, hypoxemia, and BAL fluid eosinophilia |