 Bevacizumab
Bevacizumab
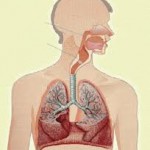
Bevacizumab, a monoclonal antibody against endothelial growth factor, has been used to treat patients with a variety of cancers. There are several reported pulmonary toxicities associated with bevaci-zumab therapy. Pulmonary hemorrhage and hemoptysis has been reported in 2.3% of patients with nonsquamous NSCLC. In these patients, pulmonary hemorrhage may lead to respiratory failure, and fatalities have been reported in 1.6% of patients treated with bevacizumab. Severe hemoptysis and pulmonary hemorrhage associated with bevacizumab therapy is more common in patients, with squamous cell carcinoma being reported in up to 31% of patients. Bevacizumab has also associated with increased risk of deep venous thrombosis (DVT) and pulmonary embolism.
Matuzumab
Matuzumab is a humanized Ig G1 that blocks the activation of EGFR. Matuzumab is active against EGFR-positive NSCLC. Bronchospasm related to matuzumab has been reported in 5% of patients. Premedication with corticosteroids is effective to prevent bronchospasm.
Trastuzumab
Trastuzumab is a humanized monoclonal antibody that selectively binds to the human EGFR (HER)-2 protein. Trastuzumab is indicated for the treatment of metastatic breast cancers with overexpression of HER-2 protein. The incidence of trastuzumab-induced pneumonitis is 0.4 to 0.6%. Trastuzumab-induced pneumonitis may present with rapidly progressive pulmonary infiltrates and respiratory failure after receiving 1 dose of trastuzumab or after 6 weeks of therapy. The mortality of trastuzumab-induced pneumonitis is about 0.1%. Acute neutrophilic alveolitis and organizing pneumonia after tras-tuzumab treatment also have been reported. Infusion-related symptoms, including hypotension, angioedema, bronchospasm, dyspnea, fever, chills, and urticaria, has been reported to occur in about 15% of patients. Severe episodes of hypotension, bronchospasm, and hypoxemia leading to death are rare.
Nucleoside Analogs
Gemcitabine
Gemcitabine is a nucleoside analog with activity against a variety of solid tumors, especially NSCLC and pancreatic cancer. A variety of forms of pulmonary toxicity have been described with the use of gemcitabine. Dyspnea developing within hours of infusion has been reported to occur in about 10% of patients. Most patients improve with therapy with diuretics and corticosteroids. Bronchospasm develops in about 0.6% of patients. These infusion-related reactions are usually mild and rarely have resulted in the discontinuation of treatment. Gemcitabine-induced pneumonitis has been reported to occur in up to 13.8% of patients. An analysis’ of pooled data from a large database showed the incidence of gemcitabine-induced pulmonary toxicity to vary from 0.02 to 0.27%. The following three types of gemcitabine-induced pneumonitis have been described: (1) a capillary leak syndrome with pulmonary edema; (2) diffuse alveolar damage; and (3) alveolar hemorrhage. Previous lung disease, chest radiation, and concurrent treatment with other agents (eg, paclitaxel, docetaxel, ifosfamide, or granulocyte colony-stimulating factor) are possible risk factors. Restrictive physiology with a marked reduction in diffusion capacity has been reported. Although the mortality rate can be as high as 20%, a rapid response (within days) to prednisone 60 mg daily (you can buy in our store prednisone), has been described in the literature.
More articles on Novel Antineoplastic Agents for Solid Tumors:
Novel Antineoplastic Agents for Solid Tumors: Podophyllotoxins
Novel Antineoplastic Agents for Solid Tumors: Taxanes